Enlarge Image
Challenging the theoretical assessment of a hypothesis on protein folding stability that has been believed for 60 years
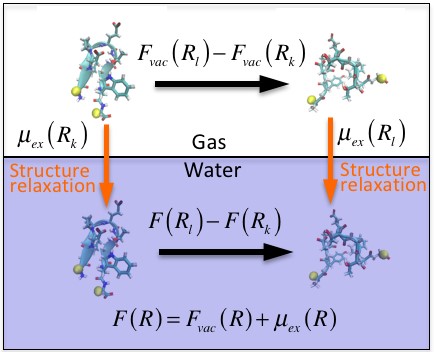
Understanding the dominant factor behind thermodynamic stability of proteins is still a challenging issue in biochemistry, biophysics, and molecular biology. Kauzmann’s hydrophobic interaction hypothesis, which considers solvation effects mediated by water as the dominant factor in thermodynamic stability of proteins, has been widely accepted for about sixty years and attracted many scientists. However, the hypothesis has not been verified or disproved because it is difficult, both theoretically and experimentally, to quantify the solvent effects on protein stability.
The theoretical physical chemistry group at the Research Institute for Interdisciplinary Science, Okayama University (Tomonari Sumi and Kenichiro Koga) developed a computational method to quantify energetics of protein folding stability and applied it to a small design protein chignolin. On the basis of results obtained for chignolin, they derived an obvious conclusion that was qualitatively different from the hypothesis: the driving force of the protein folding is attributed to the intramolecular interactions of the protein and the solvent effects rather stabilize unfolded states. These results provide new insights for strategies to design artificial proteins and developments of new biopharmaceuticals.
Reference:
- Authors: Tomonari Sumi and Kenichiro Koga.
- Title of original paper: Theoretical analysis on thermodynamic stability of chignolin.
- Journal, volume, pages and year: Scientific Reports 9, 5186 (2019).
- Digital Object Identifier (DOI): 10.1038/s41598-019-41518-1
- Journal website: https://www.nature.com/articles/s41598-019-41518-1
- Affiliations: Research Institute for Interdisciplinary Science, Okayama University.
- Department website:http://www.riis.okayama-u.ac.jp/en/
- Okayama University Scientific Achievement Repository: http://ousar.lib.okayama-u.ac.jp/57014